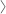Description
Cardiff, UK – 17th December 2012 – EKF Diagnostics, worldwide manufacturer of point-of-care (POC) diagnostic tools, will be exhibiting its innovative range of products for diabetes and ketosis testing at Arab Health 2013 (28 – 31st January). As the world's longest running healthcare exhibition and congress, it attracts healthcare professionals from around the world to the International Convention and Exhibition Centre in Dubai. This event will also see the first in a series of worldwide product roadshows to be undertaken by EKF during 2013.On display at stand RJ15 will be the Quo-Test and Quo-Lab glycated haemoglobin (HbA1c) analysers for near-patient measurement and monitoring of diabetes. HbA1c monitoring is increasingly used in the detection and management of diabetes and both analysers provide highly accurate, affordable and easy-to-use technology, which uses just 4 µL of venous or finger prick blood to deliver lab-accurate results within four minutes.
The success of EKF’s flagship analyser, the Quo-Test, has resulted in 1,400 units already in use and the CE marked analyser has been approved by China's SFDA for the monitoring of HbA1c in diabetes patients. In July 2012 the small and lightweight Quo-Lab HbA1c analyser was launched. Quo-Lab is designed specifically to meet the needs of clinics and laboratories in emerging economies, where diabetes is an increasingly large public health issue, and is ideal for GP surgeries, diabetes clinics and laboratories.
EKF will also be previewing its first strip-based ketone analyser, the STAT-SiteTM M HB, which uses measurements of ß-Hydroxybutyrate (HB), the main ketone produced during ketosis, to provide a quantitative and accurate assessment of patients displaying symptoms of ketosis. The technology is changing the way hospitals test for diabetic ketoacidosis and enables testing to move from the laboratory, straight into the emergency room.
The launch of STAT SiteTM M HB follows the recent success of the ß-Hydroxybutyrate (HB) LiquiColor® Reagent System developed by EKF subsidiary, Stanbio Laboratory, experts in clinical diagnostics for over 50 years and a regular exhibitor at Arab Health. The HB liquid stable reagent can be used on any open channel analyser and is used by 13 of the USA’s top 25 diabetes and endocrinology hospitals to provide rapid, objective results.
During the exhibition, EKF Diagnostics will also be running the first event in a series of worldwide roadshows planned for 2013. The roadshows will enable EKF to provide intensive training and support to its distributors, and offer a platform for important dialogue and feedback. The first event, to be held at the Shangri La Hotel in Dubai is already over-subscribed.
Julian Baines, Group CEO, “Following the overwhelming response we received at Medica we are now looking forward to showcasing our full portfolio of POC solutions at Arab Health for the first time. We are proud of our company’s expert knowledge in blood analysis, which provides cutting edge solutions that are revolutionising the way that conditions such as diabetes and ketosis are being tested, ensuring rapid diagnosis and improved patient outcomes.
We are also delighted by the response to the first of our roadshows. We reached capacity within 24 hours of announcing the event and we’ve had to find an alternative location to meet the demand. This demonstrates that our distributors are confident they can use EKF’s range to grow their own businesses in 2013.”